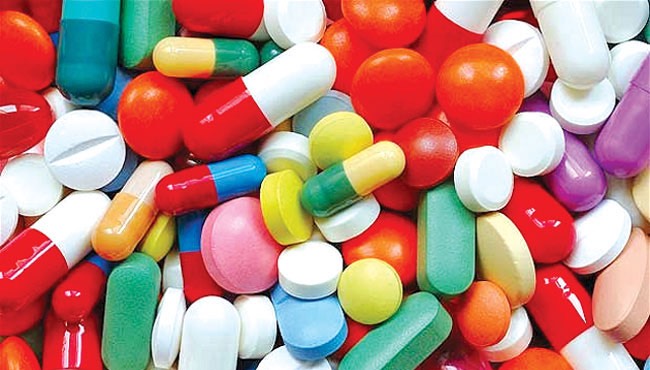
An anti-tumor drug manufactured by a Russian biotech company is registered in Sri Lanka for the treatment of cancer patients following proper guidelines and is safe for treatment, Sri Lanka’s National Medicines Regulatory Authority said.
The Ministry of Health said the National Medicines Regulatory Authority has followed the guidelines and the regulations of the European Drug Authority, when registering the biosimilar anti-tumor drug trastuzumab for cancer.
“The authority followed European Medicines Agency guidelines and scientific principles for registering the Russian manufactured trastuzumab biosimilar in Sri Lanka,” the authority said in a statement.
The authority said they always follow the due process for registration of pharmaceutical products and “biosimilars”.
The drug was approved in the country earlier this year. Distribution agreement with a partner in Sri Lanka was made in 2014.
The monoclonal antibody drug is produced by a complex biological process in bacterial cells and therefore the prices of such drugs are high. However, the expense can be reduced by having a “biosimilar” drug, the Authority explained.
The drug manufactured in Russia is used clinically to prevent breast cancer in preliminary stage and spreading of cancer cells throughout the body. Therefore, it is evident that similar results can be expected from this drug in comparison to the previous drugs as well, the Authority said.
According to the National Medicines Regulatory Authority, to date more than 100,000 vials of the biosimilar in question has been administered to patients with breast cancer in Russia, about 25 percent suffering from early stage disease, without significant safety issues.
The Ministry said India has also produced a similar drug and it has been submitted for registration as well but there have been certain clinical issues related to its overall response benefit in the clinical trial reports provided to the authority.
Therefore, the National Drug Regulatory Authority will register that drug after such issues are addressed, the Ministry of Health says.
BIOCAD, the Russian and international biotech company that manufactures the anti-tumor drug trastuzumab started delivery of the drug to Sri Lanka in April this year after the drug was approved in Sri Lanka a month before.
In July Sri Lanka’s Court of Appeals dismissed a lawsuit by the Swiss drugmaker F. Hoffmann-La Roche, Ltd. against the local distributor of BIOCAD in Sri Lanka, asking to ban the distribution of trastuzumab claiming that products by BIOCAD lacked proper clinical trials and substantial violations of the drug manufacturing process had been committed.





![TV-Poster-All-Exhibition-Sri-Lanka-in-Focus-USA-2025[1]](https://www.srilankafoundation.org/wp-content/uploads/2025/04/TV-Poster-All-Exhibition-Sri-Lanka-in-Focus-USA-20251-450x450.jpg)